Collision Theory Worksheet - Answer Key
Back to the other Chemical Kinetics Workbooks and other General Chemistry Workbooks
Go To -> Worksheet - Answer Key - Solutions Manual
- What are three requirements for a reaction to proceed?
- Particles must collide with each other.
- Particles must be correctly orientated.
- Paritcles must have enough energy to meet activation energy (Ea).
- Particles must collide with each other.
- What is Ea?
The energy required for reactantsàproducts.
- Label
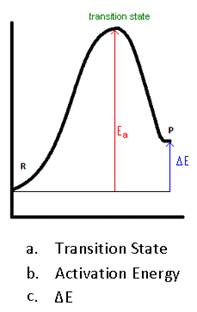
- Write the Arrhenius Equation
k = Ae(-Ea/RT)
- The activation energy for the decomposition of HI(g) to H2g) and I2(g) is 186 kJ/mol. The rate constant at 555K is 3.52 x 10-7L/mol s. What is the rate constant t 645K?
k = 9.75 x 10-5 M-1s-1
- The reaction
(CH3)3CBr + OH-→ (CH3)3COH + Br-
in a certain solvent is first order with respect to (CH3)3CBr and zero order with respect to OH-. A plot of ln(k) versus 1/T was constructed that resulted in a straight line with the slope of -1.10 x 104 K and a y intercept of 33.5.
- Determine the Ea.
Ea = 91454 J
- Determine A.
A = 3.54 x 1014
- Calculate k at 298K.
k = 0.0330 s-1
- Determine the Ea.
- Consider
NO2(g) + CO(g)→ NO(g) + CO2(g)
If the activation energy is 125 kJ/mol, and ΔE for the reaction is -216 kJ/mol. What is the activation energy for the reverse reaction?
Ea= 341 kJ/mol
